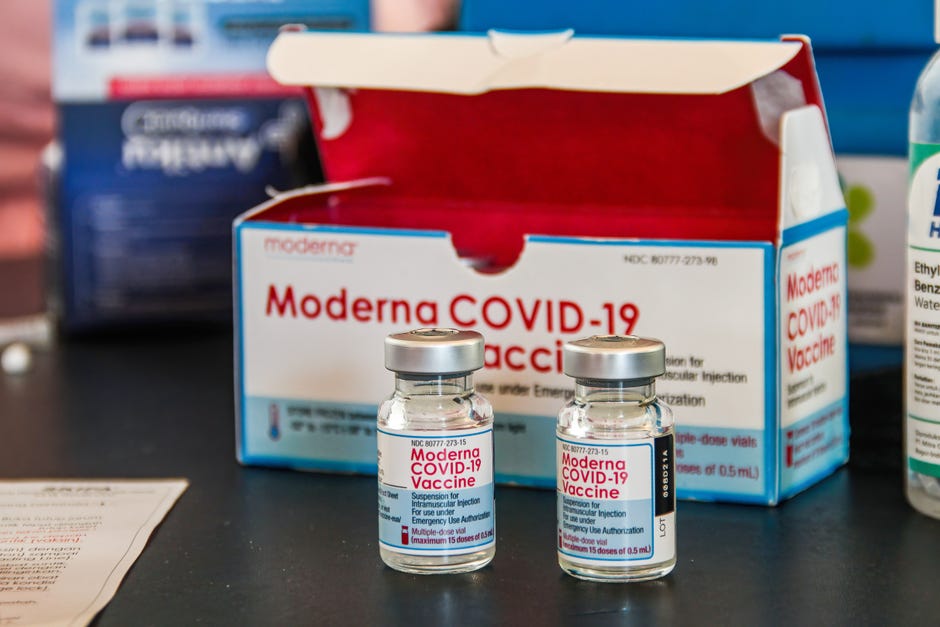
Moderna Booster Shot Release Date
Stay up to date. NasdaqMRNA a biotechnology company pioneering messenger RNA mRNA therapeutics and vaccines today announced that the US.

Covid Booster Data Shows Third Shots Not Appropriate At This Time Scientists Say
On December 18 2020 the US.
Moderna booster shot release date. People in the recommended groups who got the Moderna or JJJanssen vaccine may need a booster shot. Moderna to Develop Booster Shots to Combat Variants. A plan is underway to offer booster shots to fully vaccinated Moderna and Pfizer recipients by Sept.
For Immediate Release. More data on the effectiveness and safety of Moderna and JJJanssen booster shots are expected soon. 1 day agoTo support the authorization for emergency use of a single booster dose of the Moderna COVID-19 Vaccine the FDA analyzed immune response data from 149 participants 18 years of age.
Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. CAMBRIDGE Mass October 20 2021--Moderna Announces FDA Authorization of a Booster Dose of Modernas COVID-19 Vaccine in the US. 1 day agoThe FDA just authorized booster shots of Modernas and Johnson Johnsons COVID-19 vaccines and is letting users mix and match shots.
According to the source the FDAs advisory group will meet on. 152 153 It also started testing to see if a third shot of the existing vaccine. View press release.
FDA will undertake an independent. With those data in hand CDC will keep the public informed with a timely plan for Moderna and JJJanssen booster shots. Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept.
On the other hand Officials anticipate that authorities will make a decision on boosters for Pfizers vaccine by September 20. Learn more about the facts behind the COVID-19 Vaccine. The federal government has announced that starting late September booster shots will be made available to those who initially.
Food and Drug Administration FDA has authorized for emergency use a booster dose of the Moderna COVID-19 vaccine. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA. 14-15 where the panel will review booster data from both JJ and Moderna.
FDA supports the Administrations work to plan for the deployment of additional vaccine doses or boosters this fall. FDA authorized the emergency use of the Moderna COVID-19 Vaccine in individuals 18 years of age or older. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization.
Moderna has received emergency or other. The Moderna CEO says booster shots will be available by late summer or early fall. The Moderna CEO says booster shots will be available by late summer or early fall.
As Sanofis expected delivery date of its COVID-19 vaccine was delayed to summer 2021. On 25 January 2021 Moderna started development of a new form of its vaccine called mRNA-1273351 that could be used as a booster shot against the Beta variant lineage B1351. Learn more about the facts behind the COVID-19 Vaccine.
Pfizer Release New Vaccine Efficacy. Around two million vaccinations are now being administered each week in Australia. Moderna on Wednesday released more data on so-called breakthrough cases it says supports the push for wide use of Covid-19 vaccine booster shots.
Novavax and Moderna vaccines already secured for supply into the future Australia is well prepared to provide booster doses if they are recommended by the medical experts. Information About Booster Shots. Food and Drug Administration is announcing two upcoming meetings of its Vaccines and Related Biological Products Advisory.
Health advisers said Thursday that some Americans who received Modernas COVID-19 vaccine should get a half-dose booster to bolster protection against the virus. The booster shot meetings are slated for Oct. 20 although the Food and Drug Administration said Modernas boosters likely wont be ready.
Booster shots may be ready for the public sooner than imagined. Said in a September news release. CAMBRIDGE Mass--BUSINESS WIRE-- Moderna Inc.
Booster shot for severely immunocompromised Australians.
Moderna Vs Pfizer What We Know About Booster Shots So Far Nbc Chicago

People Are Getting Moderna Boosters Anyway Medpage Today
Latest Cdc Clears The Way For Booster Shots For Moderna And Johnson Johnson Vaccines

Covid 19 Booster Shots Are They Variant Specific
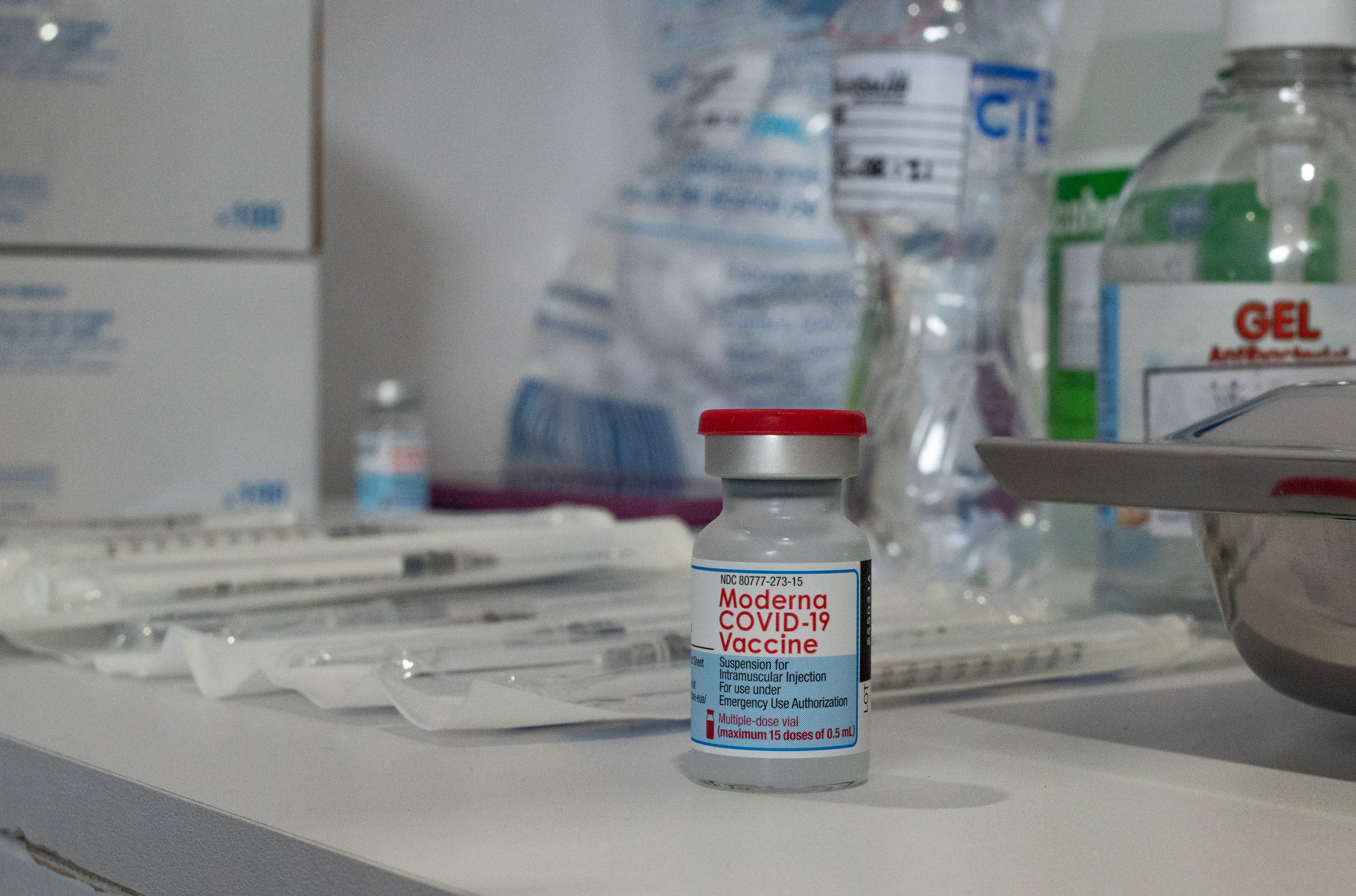
Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
Moderna Covid Vaccine Booster Shot The Latest On Approval Process Cnet

Will Canadians Need Covid Booster Shots Macleans Ca
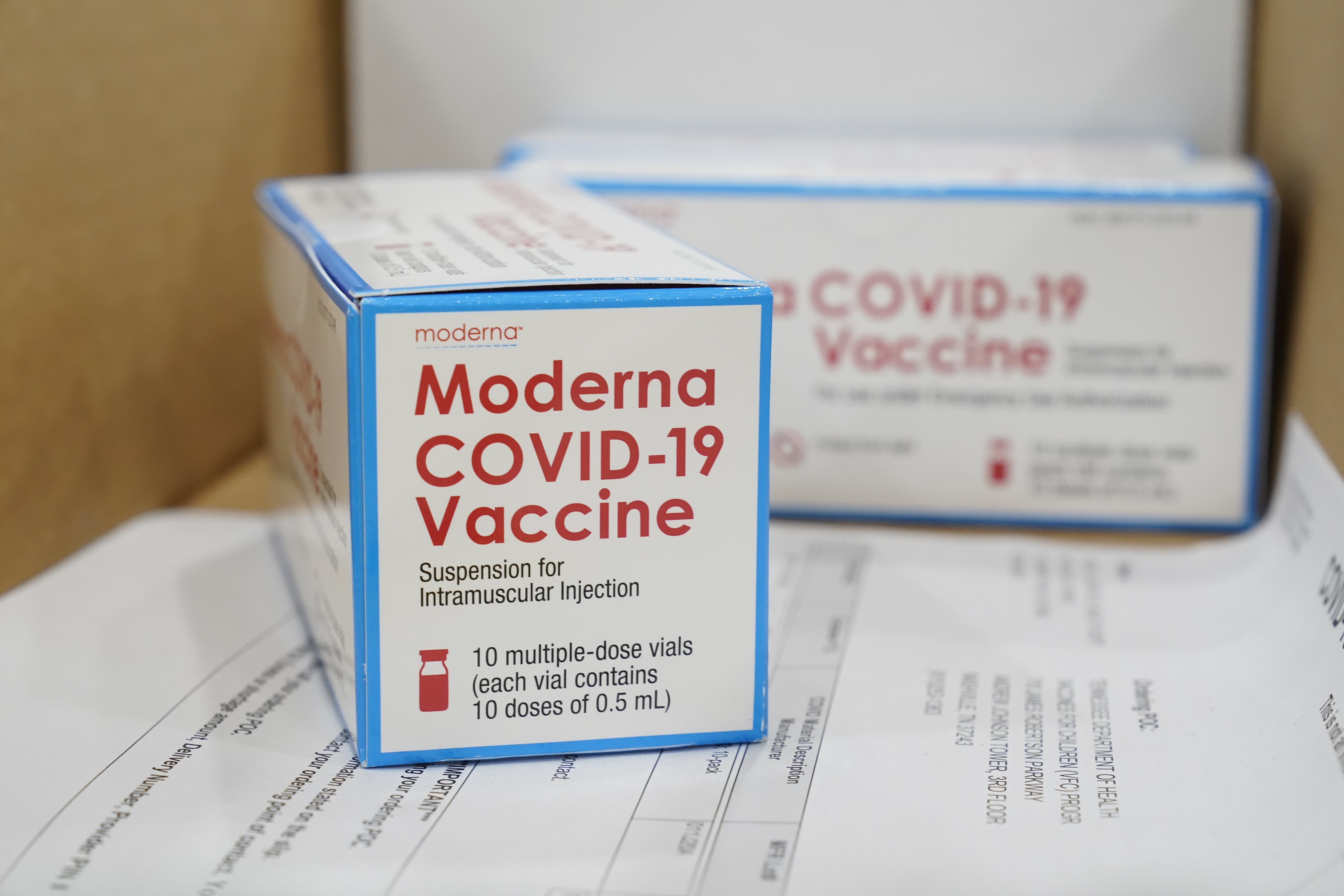
Covid Vaccine Moderna Hopes To Have Booster Shot Ready By The Fall Says Ceo

What To Know About Covid Vaccine Booster Shots The Washington Post

With Pfizer Vaccine Boosters Now Available What S Next For Moderna And J J What To Know Cnet
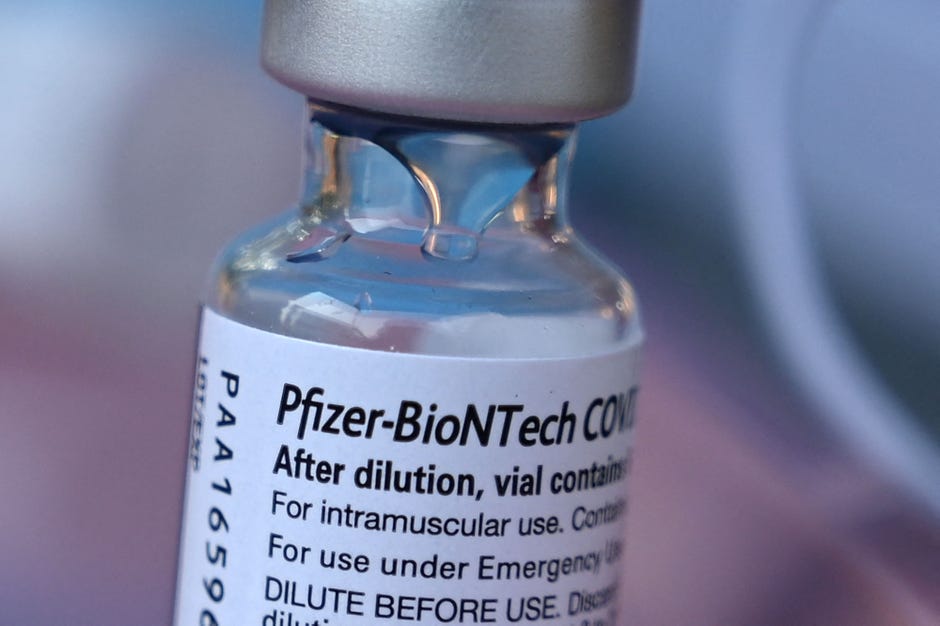
Pfizer S Covid Booster Shots Who S Eligible Vaccine Availability And More Cnet

Pfizer Covid Booster Shots Likely Ready Sept 20 Anthony Fauci Says
Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News

Booster Shots Fda Oks Extra Shots Of Pfizer Covid 19 Vaccine For Seniors Others At High Risk Abc7 Chicago

Moderna Covid Vaccine Booster Produces Robust Response Against Delta
What People Who Got J J Covid Vaccine Need To Know About Boosters
Covid 19 Vaccine Booster Shot Side Effects Similar To First Two Doses Says Cdc Cbs News
Study Shows Booster Shot After 6 To 12 Months Likely To Provide Best Protection From Covid 19 Pfizer Says Abc News